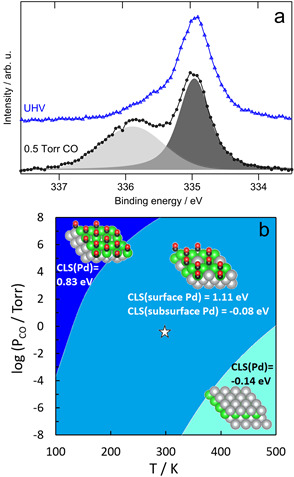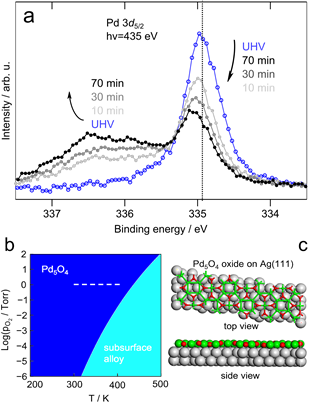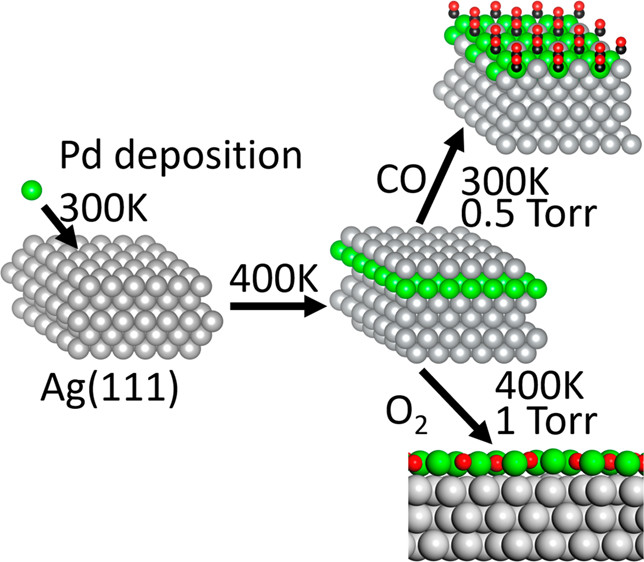
The stable form of the surface alloy in vacuum was determined to be a Ag-capped Pd-Ag surface alloy, based on a combination of X-ray photoelectron spectroscopy (XPS), scanning tunneling microscopy (STM) and density functional theory (DFT). Exposure of these Ag/PdAg/Ag(111) islands to CO (0.5 Torr) at 300 K induces migration of Pd to the surface, driven by the energetic stabilization of the Pd-CO bond based on ambient pressure XPS. Once the Pd is drawn to the surface by higher pressures of CO, it remains stable at lower temperatures (100−300 K) even under vacuum based on phase behavior modeled using DFT. Exposure to 1 Torr of O2 at 400 K also causes Pd to resurface, and, the resulting structure persists even at low pressures and temperatures below 300 K. These results establish that the state of the Pd/Ag catalyst surface depends strongly on pretreatment and operational conditions.